Daniel Crespo, professor d'Enginyeria Física, ha publicat un article a Science junt amb altres investigadors
06/09/2013
Daniel Crespo, professor del Grau en Enginyeria Física, junt amb altres investigadors, ha publicat un article a Science titulat "Element-Resolved Corrosion Analysis of Stainless-Type Glass-Forming Steels". En aquest treball han estudiat els processos de corrosió en acers amorfs, que són similars als acers inoxidables però sense estructura cristal·lina. La seva elevada resistència a la corrosió permetria emprar-los en bateries de Li-ion, que es fan servir actualment en els 'smart phone', augmentant-ne la durada.
Per a una informació una mica més detallada cliqueu aquí.
Aquesta notícia s'ha publicat a la Sala de Premsa de la UPC.
The rusting of iron and steel can be prevented through the addition of 11% or more chromium. The addition of molybdenum can enhance the corrosion resistance, with a complex interplay between the Cr and Mo atoms. However, if chemical variations exist, corrosion can still occur in localized regions or if the surface layer is mechanically abraded. The authots of this article studied the corrosive failure of an iron-based glassy alloy. A combination of atom probe tomography, electron microscopy, and x-ray diffraction was used to build up a near atomistic picture of local variations in the metal as it was heated and allowed to crystallize, and the impact these processes have on the corrosion resistance.
Abstract
Ultrathin passive films effectively prevent the chemical attack of stainless steel grades in corrosive environments; their stability depends on the interplay between structure and chemistry of the constituents iron, chromium, and molybdenum (Fe-Cr-Mo). Carbon (C), and eventually boron (B), are also important constituents of steels, although in small quantities. In particular, nanoscale inhomogeneities along the surface can have an impact on material failure but are still poorly understood. Addressing a stainless-type glass-forming Fe50Cr15Mo14C15B6 alloy and using a combination of complementary high-resolution analytical techniques, we relate near-atomistic insights into increasingly inhomogeneous nanostructures with time- and element-resolved dissolution behavior. The progressive elemental partitioning on the nanoscale determines the degree of passivation. A detrimental transition from Cr-controlled passivity to Mo-controlled breakdown is dissected atom by atom, demonstrating the importance of nanoscale knowledge for understanding corrosio
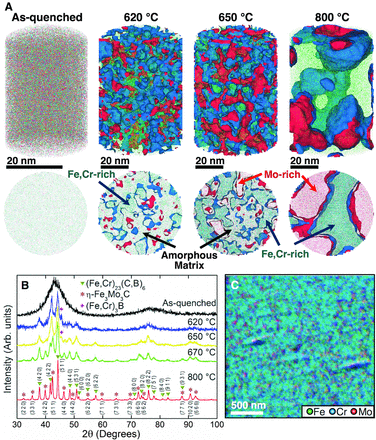 A) APT reconstructions showing the distribution of the metallic elements (Fe, green; Cr, blue; Mo, red) in the as-quenched, partially and fully crystallized alloys. Isoconcentration surfaces of 22 atomic % Mo in red and 16 atomic % Cr in blue highlight three different regions in the samples annealed for 20 min at 620° and 650°C. Thirty-two atomic % Mo and 15 atomic % Cr isoconcentration surfaces are plotted for the crystallized alloy (60 min at 800°C). Lower images are 2- to 5-nm thick virtual slices of the respective reconstructions revealing the nanoscale partitioning characteristics. (B) XRD plots indicating the formation of the different phases. (C) Overlap of the individual Fe, Cr, and Mo SAEM maps after crystallization at 800°C.
A) APT reconstructions showing the distribution of the metallic elements (Fe, green; Cr, blue; Mo, red) in the as-quenched, partially and fully crystallized alloys. Isoconcentration surfaces of 22 atomic % Mo in red and 16 atomic % Cr in blue highlight three different regions in the samples annealed for 20 min at 620° and 650°C. Thirty-two atomic % Mo and 15 atomic % Cr isoconcentration surfaces are plotted for the crystallized alloy (60 min at 800°C). Lower images are 2- to 5-nm thick virtual slices of the respective reconstructions revealing the nanoscale partitioning characteristics. (B) XRD plots indicating the formation of the different phases. (C) Overlap of the individual Fe, Cr, and Mo SAEM maps after crystallization at 800°C.
Per a una informació una mica més detallada cliqueu aquí.
Aquesta notícia s'ha publicat a la Sala de Premsa de la UPC.
The rusting of iron and steel can be prevented through the addition of 11% or more chromium. The addition of molybdenum can enhance the corrosion resistance, with a complex interplay between the Cr and Mo atoms. However, if chemical variations exist, corrosion can still occur in localized regions or if the surface layer is mechanically abraded. The authots of this article studied the corrosive failure of an iron-based glassy alloy. A combination of atom probe tomography, electron microscopy, and x-ray diffraction was used to build up a near atomistic picture of local variations in the metal as it was heated and allowed to crystallize, and the impact these processes have on the corrosion resistance.
Abstract
Ultrathin passive films effectively prevent the chemical attack of stainless steel grades in corrosive environments; their stability depends on the interplay between structure and chemistry of the constituents iron, chromium, and molybdenum (Fe-Cr-Mo). Carbon (C), and eventually boron (B), are also important constituents of steels, although in small quantities. In particular, nanoscale inhomogeneities along the surface can have an impact on material failure but are still poorly understood. Addressing a stainless-type glass-forming Fe50Cr15Mo14C15B6 alloy and using a combination of complementary high-resolution analytical techniques, we relate near-atomistic insights into increasingly inhomogeneous nanostructures with time- and element-resolved dissolution behavior. The progressive elemental partitioning on the nanoscale determines the degree of passivation. A detrimental transition from Cr-controlled passivity to Mo-controlled breakdown is dissected atom by atom, demonstrating the importance of nanoscale knowledge for understanding corrosio
 A) APT reconstructions showing the distribution of the metallic elements (Fe, green; Cr, blue; Mo, red) in the as-quenched, partially and fully crystallized alloys. Isoconcentration surfaces of 22 atomic % Mo in red and 16 atomic % Cr in blue highlight three different regions in the samples annealed for 20 min at 620° and 650°C. Thirty-two atomic % Mo and 15 atomic % Cr isoconcentration surfaces are plotted for the crystallized alloy (60 min at 800°C). Lower images are 2- to 5-nm thick virtual slices of the respective reconstructions revealing the nanoscale partitioning characteristics. (B) XRD plots indicating the formation of the different phases. (C) Overlap of the individual Fe, Cr, and Mo SAEM maps after crystallization at 800°C.
A) APT reconstructions showing the distribution of the metallic elements (Fe, green; Cr, blue; Mo, red) in the as-quenched, partially and fully crystallized alloys. Isoconcentration surfaces of 22 atomic % Mo in red and 16 atomic % Cr in blue highlight three different regions in the samples annealed for 20 min at 620° and 650°C. Thirty-two atomic % Mo and 15 atomic % Cr isoconcentration surfaces are plotted for the crystallized alloy (60 min at 800°C). Lower images are 2- to 5-nm thick virtual slices of the respective reconstructions revealing the nanoscale partitioning characteristics. (B) XRD plots indicating the formation of the different phases. (C) Overlap of the individual Fe, Cr, and Mo SAEM maps after crystallization at 800°C.
Comparteix: