Ernest Mendoza, professor d'Enginyeria Física, ha publicat un article a Nature junt amb altres investigadors
30/08/2013
Ernest Mendoza, professor del Grau en Enginyeria Física, junt amb altres investigadors, ha publicat un article a Nature Chemistry titulat "Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity", on demostren com el control de la mida de petites agrupacions o clústers d'or (de 5 a 10 àtoms) augmenta excepcionalment la seva capacitat catalitzadora, la qual cosa podria tenir aplicacions en nombrosos sectors industrials. Ernest Mendoza, del Departament de Física i Enginyeria Nuclear (DFEN) de la UPC, treballa al Laboratori de Nanomaterials Aplicats del Centre de Recerca en Nanoenginyeria (CRnE).
Aquesta notícia s'ha publicat a la Sala de Premsa de la UPC.
Abstract
The catalytic activity of gold depends on particle size, with the reactivity increasing as the particle diameter decreases. However, investigations into behaviour in the subnanometre regime (where gold exists as small clusters of a few atoms) began only recently with advances in synthesis and characterization techniques. Here we report an easy method to prepare isolated gold atoms supported on functionalized carbon nanotubes and their performance in the oxidation of thiophenol with O2. We show that single gold atoms are not active, but they aggregate under reaction conditions into gold clusters of low atomicity that exhibit a catalytic activity comparable to that of sulfhydryl oxidase enzymes. When clusters grow into larger nanoparticles, catalyst activity drops to zero. Theoretical calculations show that gold clusters are able to activate thiophenol and O2 simultaneously, and larger nanoparticles are passivated by strongly adsorbed thiolates. The combination of both reactants activation and facile product desorption makes gold clusters excellent catalysts.
Aquesta notícia s'ha publicat a la Sala de Premsa de la UPC.
Abstract
The catalytic activity of gold depends on particle size, with the reactivity increasing as the particle diameter decreases. However, investigations into behaviour in the subnanometre regime (where gold exists as small clusters of a few atoms) began only recently with advances in synthesis and characterization techniques. Here we report an easy method to prepare isolated gold atoms supported on functionalized carbon nanotubes and their performance in the oxidation of thiophenol with O2. We show that single gold atoms are not active, but they aggregate under reaction conditions into gold clusters of low atomicity that exhibit a catalytic activity comparable to that of sulfhydryl oxidase enzymes. When clusters grow into larger nanoparticles, catalyst activity drops to zero. Theoretical calculations show that gold clusters are able to activate thiophenol and O2 simultaneously, and larger nanoparticles are passivated by strongly adsorbed thiolates. The combination of both reactants activation and facile product desorption makes gold clusters excellent catalysts.
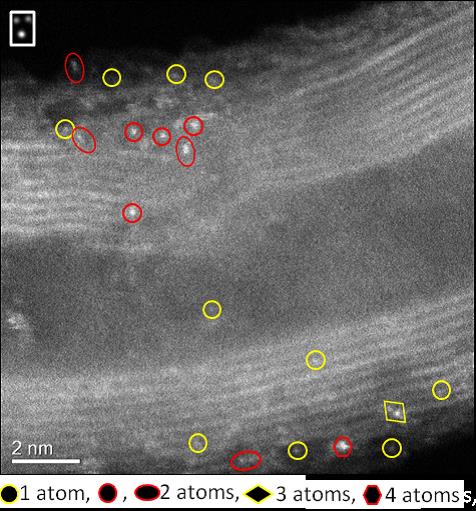
Image of sample showing the presence of gold atoms and dimers
Comparteix: